ADCs
The special class of biotherapeutics known as antibody-drug conjugates (ADC) is distinguishable based on the unique specificity of a monoclonal antibody (mAb) covalently linked to a highly potent drug payload (warhead) by an enzyme cleavable linker. ADCs are efficacious therapeutics in the treatment of oncological diseases as they specifically target certain antigens expressed on the cancer cells. ADCs enable low drug dosages and minimize systemic toxicity because of the specific affinity to the cancer cells and high potency of the warhead. Consequently, ADCs provide more potent chemotherapeutic drugs with a more tolerable side-effect profile for patients. Currently, four ADCs have been approved for use as drug products and many more are in the development and approval pipelines. Quality production of ADCs involves understanding the structural characteristics of the mAb vehicle and the combined complex itself as they impact specificity and stability of the ADC complex. Thus, there is need for characterizing the antibody portion of the complex pre-conjugation and comparing it with the complex post-conjugation to determine if critical secondary structures within the mAb have changed as they may compromise specific binding to a designated target cancer antigen.
The novel Aurora and Apollo utilize a quantum cascade laser (QCL) and a microfluidic flow cell to provide secondary structural characterization of the native antibody pre- and post-conjugation via Microfluidic Modulation Spectroscopy (MMS). This unique approach to IR spectroscopy provides confidence in the ‘similarity’ of the conjugated and unconjugated antibodies by comparing their intrinsic IR spectra. The improved sensitivity provided by the QCL and the real time background buffer subtraction powered by the MMS technology enable the ultrasensitive detection of the smallest differences between the native, warhead-free antibody, and the loaded antibody-drug conjugate.
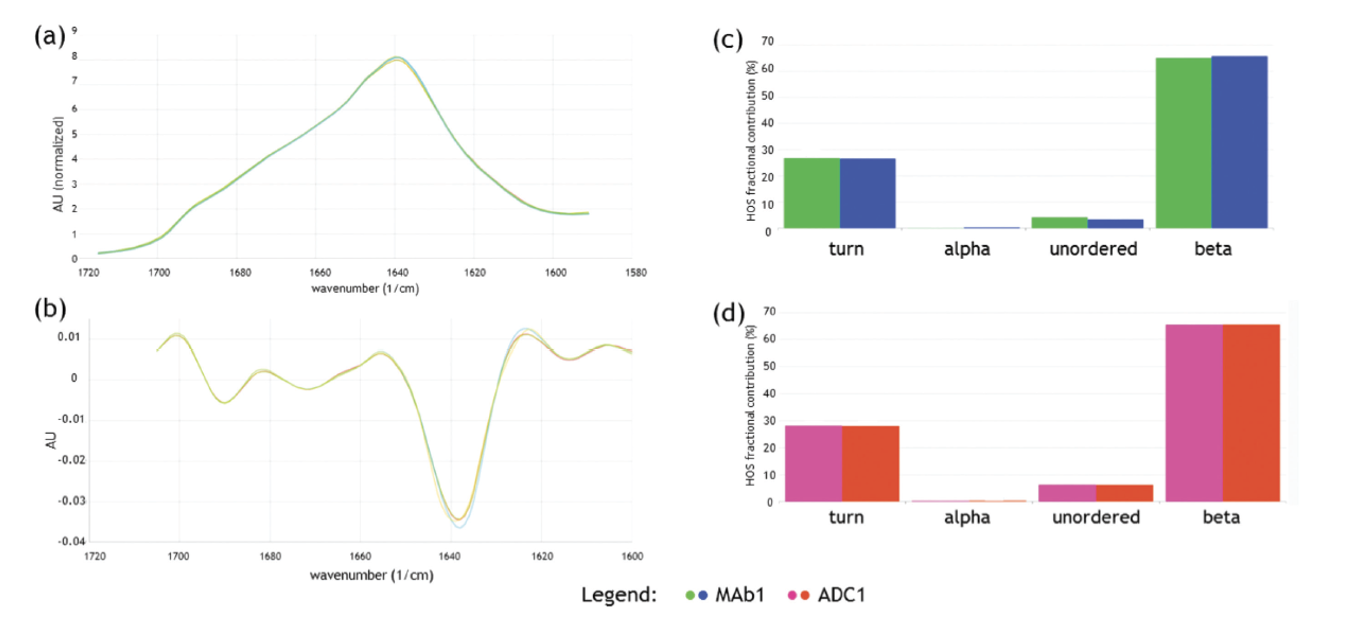
As an example of the impact of MMS and our first-generation instrument, the AQS³pro, for characterizing ADCs and their native antibodies, we carried out a series of studies in collaboration with Immunogen to demonstrate its use as a tool for ADC development. The ADC conjugating process attaches the drug to unmodified lysine residues across the mAb and the aim of this study was to confirm that the structure of the antibody had remained intact after being conjugated. The secondary structure of both native and conjugated mAbs were measured, and Figure 1 shows that the process of conjugating the warhead to the mAb does not affect the secondary structure. Lastly, the delta software was used to determine the fractional contributions of secondary structural motifs. The results were consistent with no structural change in the mAb before and after lysine-conjugation with the potent payload.